This is “Organic Nomenclature II: Alkenes, Alkynes and Benzene Derivatives”, section 8.11 from the book Principles of General Chemistry (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there.
8.11 Organic Nomenclature II: Alkenes, Alkynes and Benzene Derivatives
Learning Objectives
- To write IUPAC names for alkenes, alkynes and benzene derivatives.
- Given IUPAC systematic names of alkenes, alkynes and substituted benzenes write their structural formulas.
- To recognize stereoisomers of organic compounds.
Recall from sectiion 4.12, Organic Nomenclature I: Alkanes, Cycloalkanes and Substituents we learned the systematic nomenclature for the alkane families of organic compounds. The alkanes were hydrocarbons (contain only carbon and hydrogen) that involve only single bonds. We saw how a systematic name has several parts that can be classified as endings or prefixes. In this section we will learn the nomenclature associated with other hydrocarbon families. The good news for this section is that very little changes. We'll add a couple of new endings and a couple of new prefixes. The new prefixes are needed to distinguish a new type of isomer called stereoisomers.
The Parts
Ending: Recall the ending designates the functional group present in the molecule.
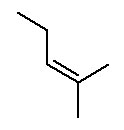
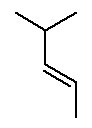
- Alkenes contain at least one C=C (carbon-carbon double bond). Alkenes end in -ene . The ending is preceded by a number indicating where the double bond is located.
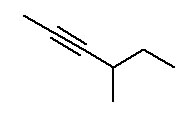
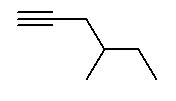
- Alkynes contain at least one C≡C (carbon-carbon triple bond). Alkynes end in -yne . The ending is preceded by a number indicating where the triple bond is located.
- Benzenes contain a benzene ring (shown below) which is six carbon atoms joined in a ring with a double bond on every carbon. Benzenes end in -benzene .
Prefix(es): Any number of prefixes can be applied to the ending.
- The prefix immediately preceding the ending indicates the number of carbons in the parent chain.
- The prefix "cyclo" indicates a ring structure.
- Prefixes, usually accompanied by numbers, are used to indicate branches in the carbon chain.
- Prefixes, again usually accompanied by numbers, are used to indicate halogen atoms that have been substituted for hydrogens.
- A prefix of cis or trans is applied to indicate the stereochemistry around the double bond of alkenes.
Note the Pattern
The only new prefix is the cis/trans designation for alkenes; otherwise, it's the same prefixes as we encountered in section 4.12.
Alkenes
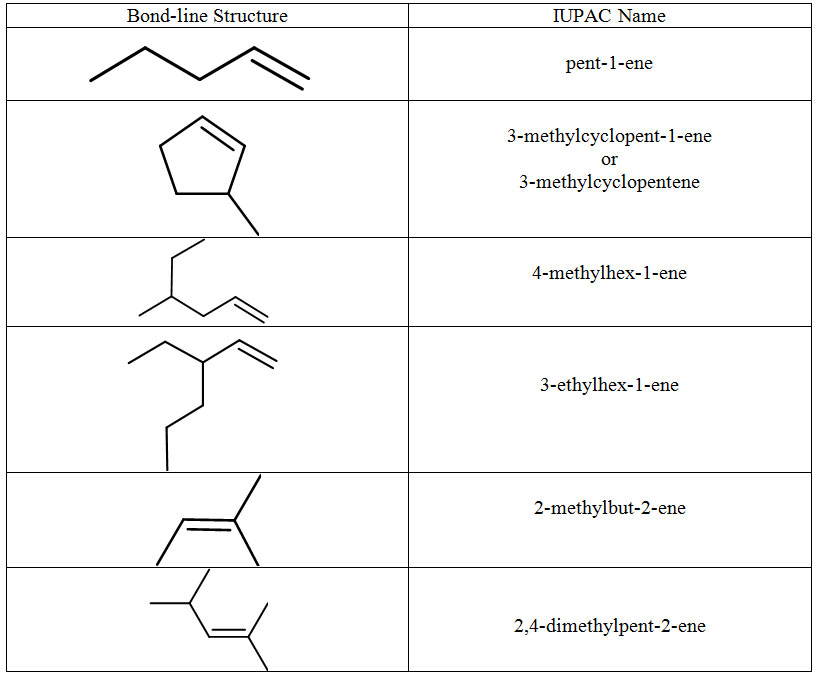
The parent chain is the longest continuous chain of carbon atoms which includes the C=C. This is not necessarily the longest chain of carbon atoms. The lowest possible number is assigned to the double bond. Counting the rest of the chain always proceeds through the double bond. Below are some named structures of alkenes.
Figure 8.15 Representative Alkenes
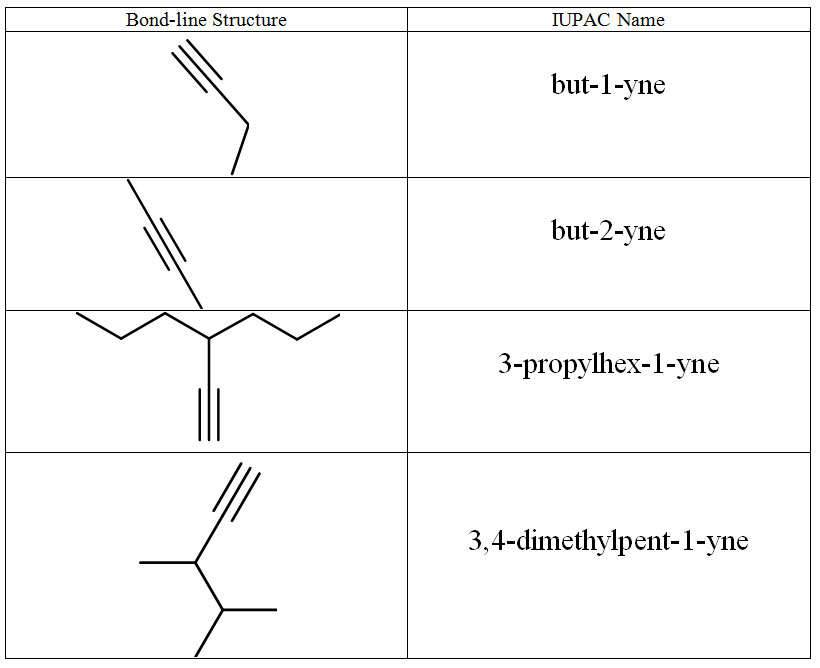
Alkynes
The same rules used for alkenes are used for alkynes. The geometry around the triple bond presents 180° bond angles which are difficult to include in a ring.
Figure 8.16 Representative Alkynes
Benzene Derivatives
Benzene has a hydrogen covalently bonded to each of the six carbons in the benzene ring (above). In the place of any of the hydrogens different groups can be substituted. The substituted benzenes are called benzene derivatives. Below are some representative benzene structures and their names.
Table 8.17 Representative Benzene Derivatives
Stereoisomers
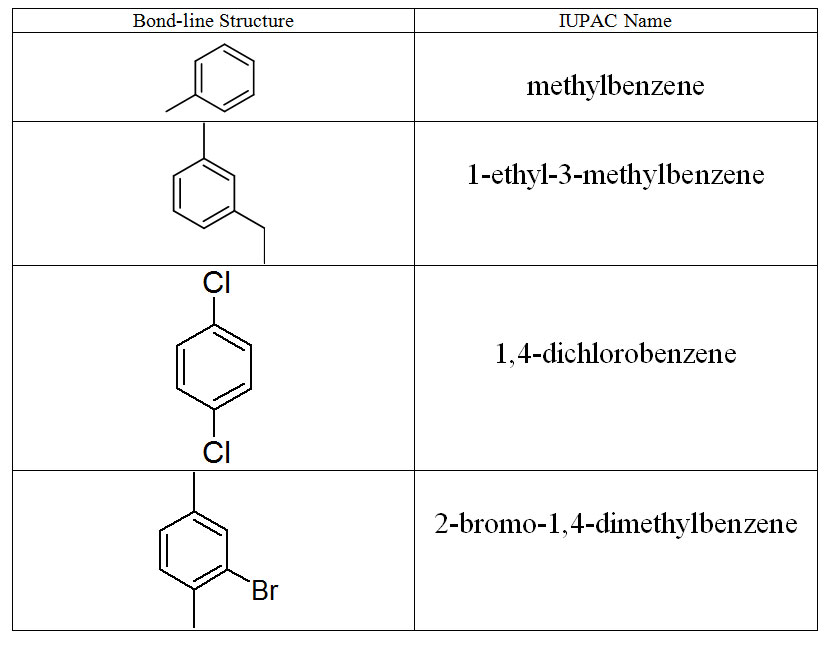
In section 4.12, Organic Nomenclature I, we defined structural isomers as different compounds that have the same molecular formula but a different connectivity of the atoms. Stereoisomers are also different compounds that have the same molecular formula but also the same connectivity of the atoms. The only difference between stereoisomers is the arrangement of the atoms in space. To designate different stereoisomers chemists can use the cis/trans system. To use the cis/trans system, one considers the two carbons of the C=C double bond. Since the valence of carbon is 4, each of those two carbons have two other bonds. The first condition to using the cis/trans system is that each of the carbons of the double bond has two different groups (or atoms) attached to it. For example but-1-ene can not have cis-trans isomers since carbon 1 has two hydrogens bonded to it shown in red below
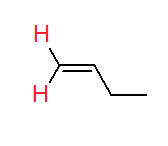
The second condition for using cis/trans is that there is a group on one of the carbons of the double bond that is also present on the other carbon of the double bond. The case of but-2-ene (below) presents exactly this situation.
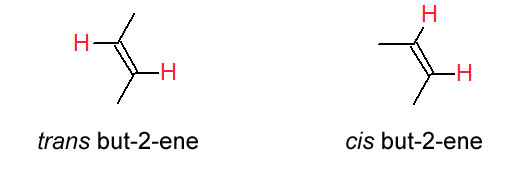
The designation trans is made when the identical groups are on the opposite side of an imaginary plane perpendicular to the real plane of atoms containing the double bond. The designation cis is made when the identical groups are on the same side of this imaginary plane. Be sure you see these two stereoisomers of but-2-ene are NOT structural isomers. Structural isomers must have different connectivity of the atoms; whereas, stereoisomers must have the same connectivity. Both cis and trans but-2-ene have exactly the same connectivity of the atoms. Cis but-2-ene and trans but-2-ene have a different arrangment of the atoms in space since the four positions around the double bond are held in this plane by the C=C. The C=C does not permit free rotation about the double bond. A C-C bond allows for free rotation so we don't need to consider cis & trans possibilities for any other functional group (yet, anyway).
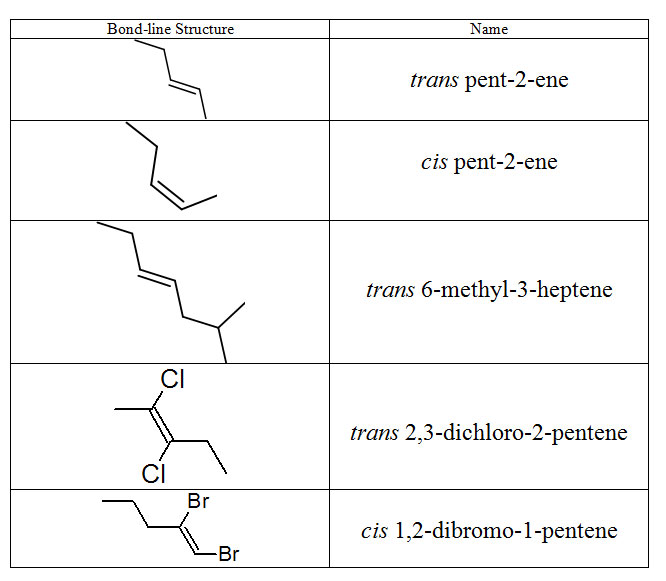
Below are some examples of naming alkenes with the cis/trans system.
Figure 8.18 Representative Cis- and Trans-Named Alkenes
Be aware this cis/trans system isn't perfect; however, cis/trans is relatively simple to use. The cis/trans convention is widely understood and even part of our popular media. We've all heard of the evils of trans fat. Cis/trans can be arbitrary in designating the stereoarrangement of the atoms. The IUPAC has another system called the E/Z system but despite its name, it's not so easy. You will learn the E/Z system in organic chemistry.
Numerical Problems
-
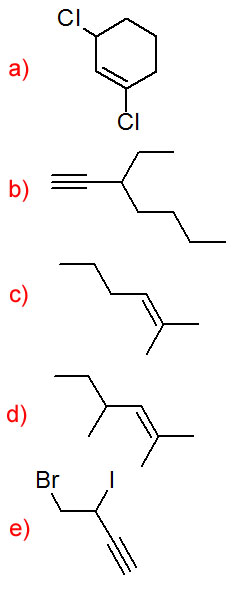
Draw a structural formula for each compound.
- 1,3-dichlorocyclohexene
- 3-ethylhept-1-yne
- 2-methylhex-2-ene
- 2,4-dimethylhex-2-ene
- 4-bromo-3-iodobut-1-yne
-
Draw a structural formula for each compound.
- cis hex-3-ene
- trans 2,5-dimethylhex-3-ene
- trans 3-methylpent-2-ene
- cis 2,2,5-trimethylhept-3-ene
- cis 2,3-dichlorobut-2-ene
-
Which of the following compounds have cis/trans isomers?
- hex-2-ene
- hex-2-yne
- cyclohexene
- hex-1-ene
- hex-3-ene
-
Name each compound.
- CH2=CHCH(CH3)CH2CH2CH3
- CH≡CCH(CH3)2
- CH3CH2CHBrCH=CH2
- CH2=CHCBr3
- (CH3)2C=CHCH3
-
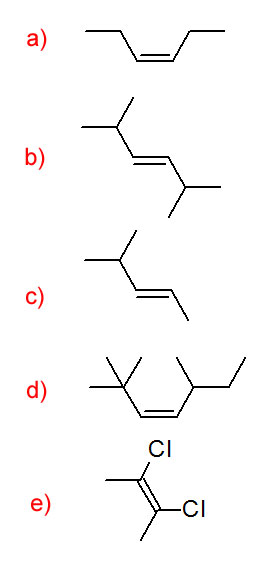
Name each compound.
-
Answers
-
-
-
- yes
- no
- no
- no
- yes
-
- 3-methylhex-1-ene
- 3-methylbut-1-yne
- 3-bromopent-1-ene
- 3,3,3-tribromoprop-1-yne
- 2-methylbut-2-ene
-
- 4-methylhex-2-yne
- 4-methylhex-1-ene
- 2-methylpent-2-ene
- trans 4-methylpent-2-ene
- trans pent-2-ene