This is “End-of-Chapter Material”, section 8.10 from the book Principles of General Chemistry (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there.
8.10 End-of-Chapter Material
Application Problems
-
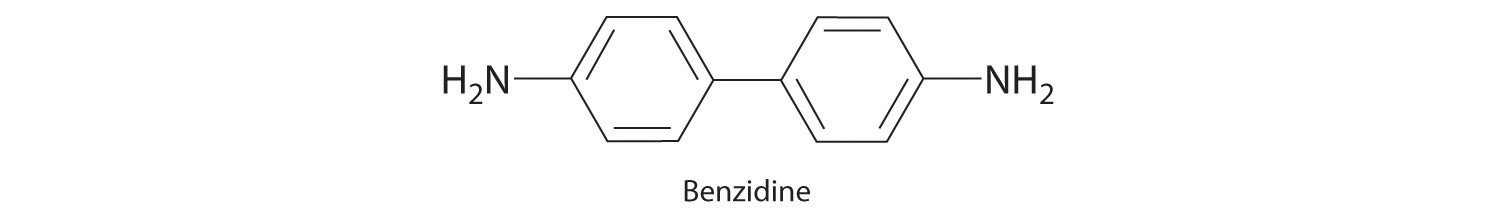
Until recently, benzidine was used in forensic medicine to detect the presence of human blood: when mixed with human blood, benzidine turns a characteristic blue color. Because benzidine has recently been identified as a carcinogen, other indicators have replaced it. Draw the complete Lewis dot structure for benzidine. Would you expect this compound to behave as a Lewis acid or a Lewis base?
-
There are three possible ways to connect carbon, nitrogen, and oxygen to form a monoanion: CNO−, CON−, and OCN−. One is the cyanate ion, a common and stable species; one is the fulminate ion, salts of which are used as explosive detonators; and one is so unstable that it has never been isolated. Use Lewis electron structures and the concept of formal charge to determine which isomer is cyanate, which is the fulminate, and which is the least stable.
-
The colorless gas N2O4 is a deadly poison that has been used as an oxidizing agent in rocket fuel. The compound has a single N–N bond, with a formal charge of +1 on each nitrogen atom. Draw resonance structures for this molecule.
-
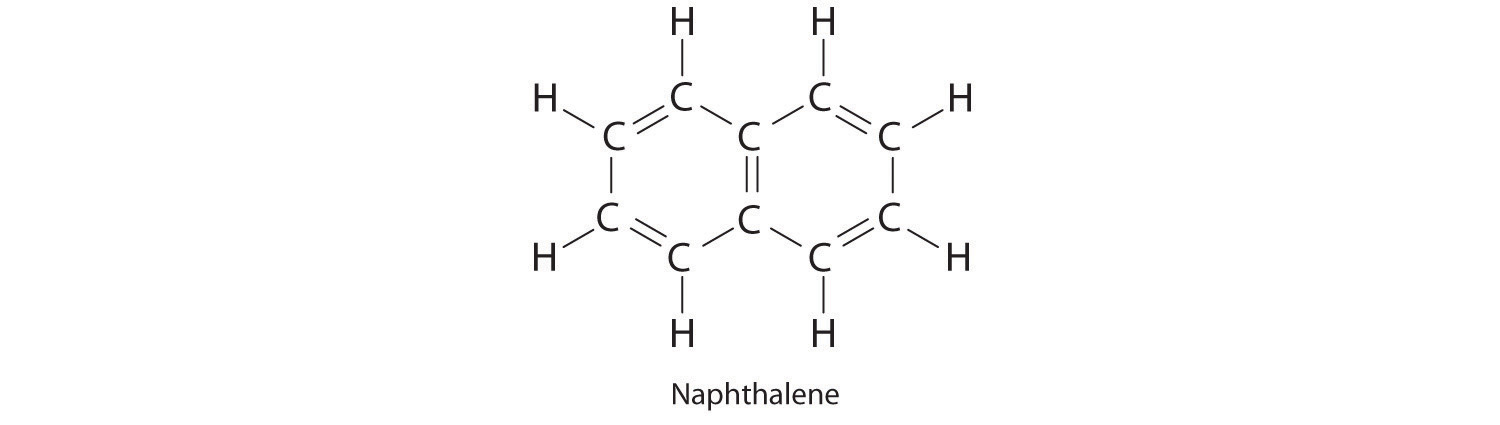
Naphthalene is an organic compound that is commonly used in veterinary medicine as the active ingredient in dusting powders; it is also used internally as an intestinal antiseptic. From its chemical structure and the ΔHf of CO2 and H2O, estimate the molar enthalpy of combustion and the enthalpy of formation of naphthalene.
-
♦ Compare the combustion of hydrazine (N2H4), which produces nitrogen and water, with the combustion of methanol. Use the chemical structures to estimate which has a higher heat of combustion. Given equal volumes of hydrazine (d = 1.004 g/mL) and methanol (d = 0.791 g/mL), which is the better fuel (i.e., which provides more energy per unit volume during combustion)? Can you think of a reason why hydrazine is not used in internal combustion engines?
-
♦ Race car drivers frequently prefer methanol to isooctane as a fuel. Is this justified based on enthalpies of combustion? If you had a choice between 10 gal of methanol (d = 0.791 g/mL) and the same volume of isooctane (d = 0.688 g/mL), which fuel would you prefer?
-
♦ An atmospheric reservoir species is a molecule that is rather unreactive, but it contains elements that can be converted to reactive forms. For example, chlorine nitrate (ClONO2) is a reservoir species for both chlorine and nitrogen dioxide. In fact, most of the chlorine in the atmosphere is usually bound up in chlorine nitrate as a result of the reaction of ClO with NO2.
- Write a balanced chemical equation for this reaction.
-
Draw Lewis electron structures for each species in this reaction. What difficulty is associated with the structure of the reactants? How does this affect the reactivity of the compounds?
Chlorine nitrate can react in a surface reaction with water to form HClO and nitric acid.
- Draw Lewis electron structures to describe this reaction.
- Identify the Lewis and Brønsted–Lowry acids.
-
♦ Aniline is an oily liquid used to prepare organic dyes, varnishes, and black shoe polishes.
- Draw a complete Lewis structure for the molecule (including the nitrogen atom).
-
The –NH2 bound to the ring contains a lone pair of electrons that can participate in resonance in the following way:
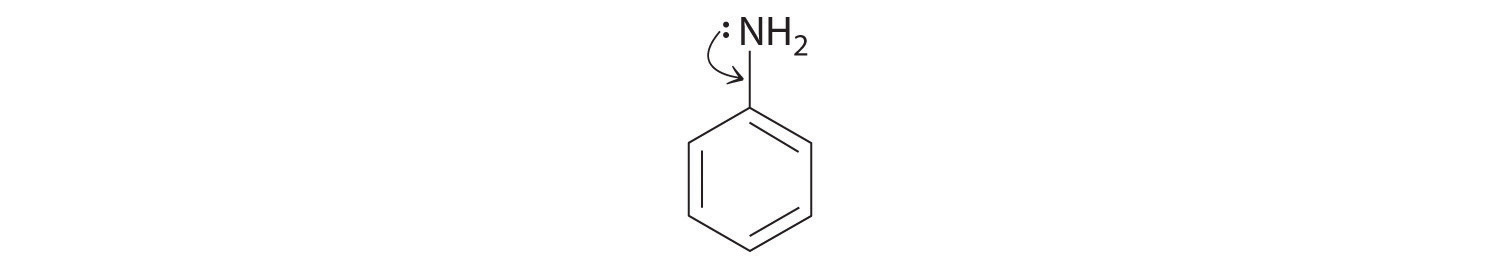
Draw a second Lewis structure for aniline that takes this interaction into account.
- Calculate the formal charge on each nonhydrogen atom in both Lewis structures.
- What other resonance structures can be drawn for aniline that satisfy the octet rule?
Problems marked with a ♦ involve multiple concepts.
Answers
-
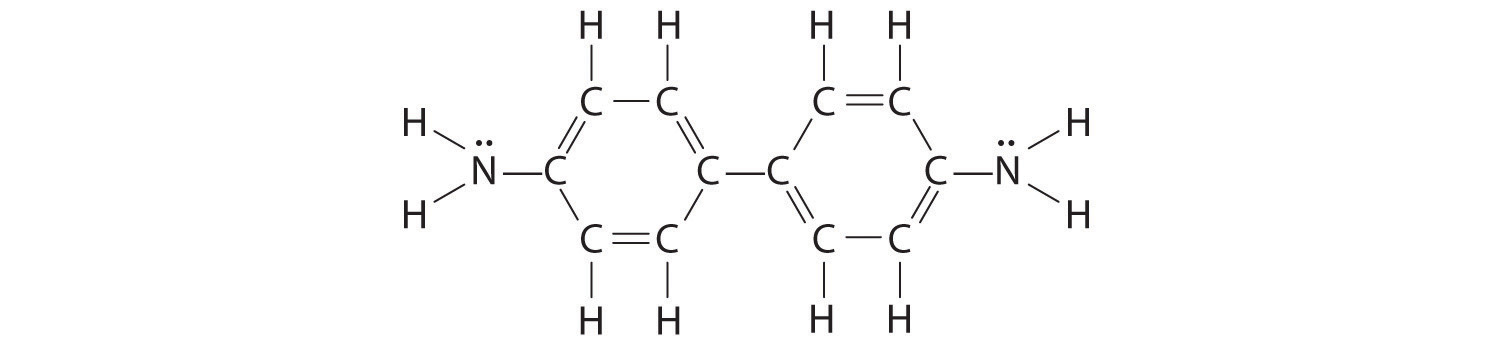
This molecule is likely to serve as a Lewis base because of the lone pair of electrons on each nitrogen atom.
-
-
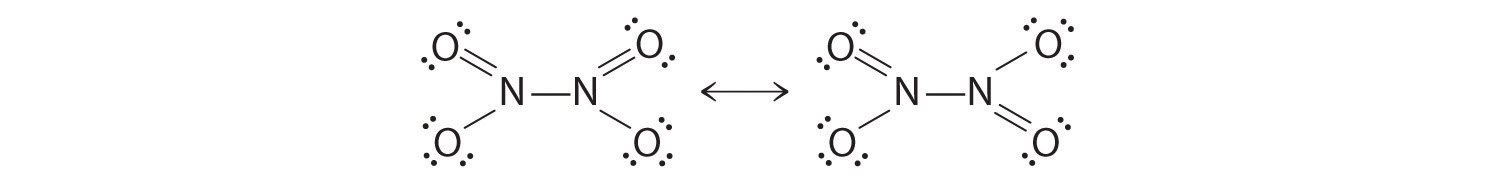
-
-
The balanced chemical reaction is:
N2H4 + O2 → N2 + 2 H2OHydrazine:
ΔHcomb = −573 kJ/mol or −17.9 kJ/mlMethanol (CH3OH):
ΔHcomb = −1286 kJ/mol or –15.9 kJ/mlHydrazine is both extremely toxic and potentially explosive.
-
-
- ClO + NO2 → ClONO2
-
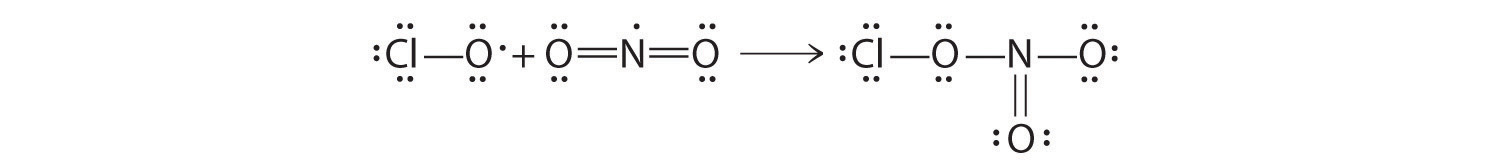
Both reactants have one unpaired electron, which makes them more reactive than might otherwise be expected.
-
ClONO2 + H2O → HClO + HONO2
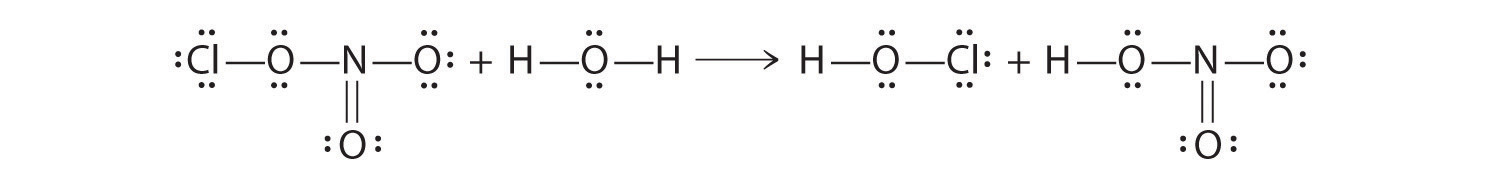
- Water is acting as a Lewis base, as well as a Brønsted–Lowry acid. A lone pair on oxygen is used to attack the N atom of chlorine nitrate, and an H+ of water is transferred to the ClO−. Chlorine nitrate acts as a Lewis acid, and OH− is transferred to NO2+, which acts as both a Lewis and a Brønsted–Lowry acid.
-




