This is “Appendix H: Periodic Table of Elements”, appendix 8 from the book Principles of General Chemistry (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there.
Chapter 32 Appendix H: Periodic Table of Elements
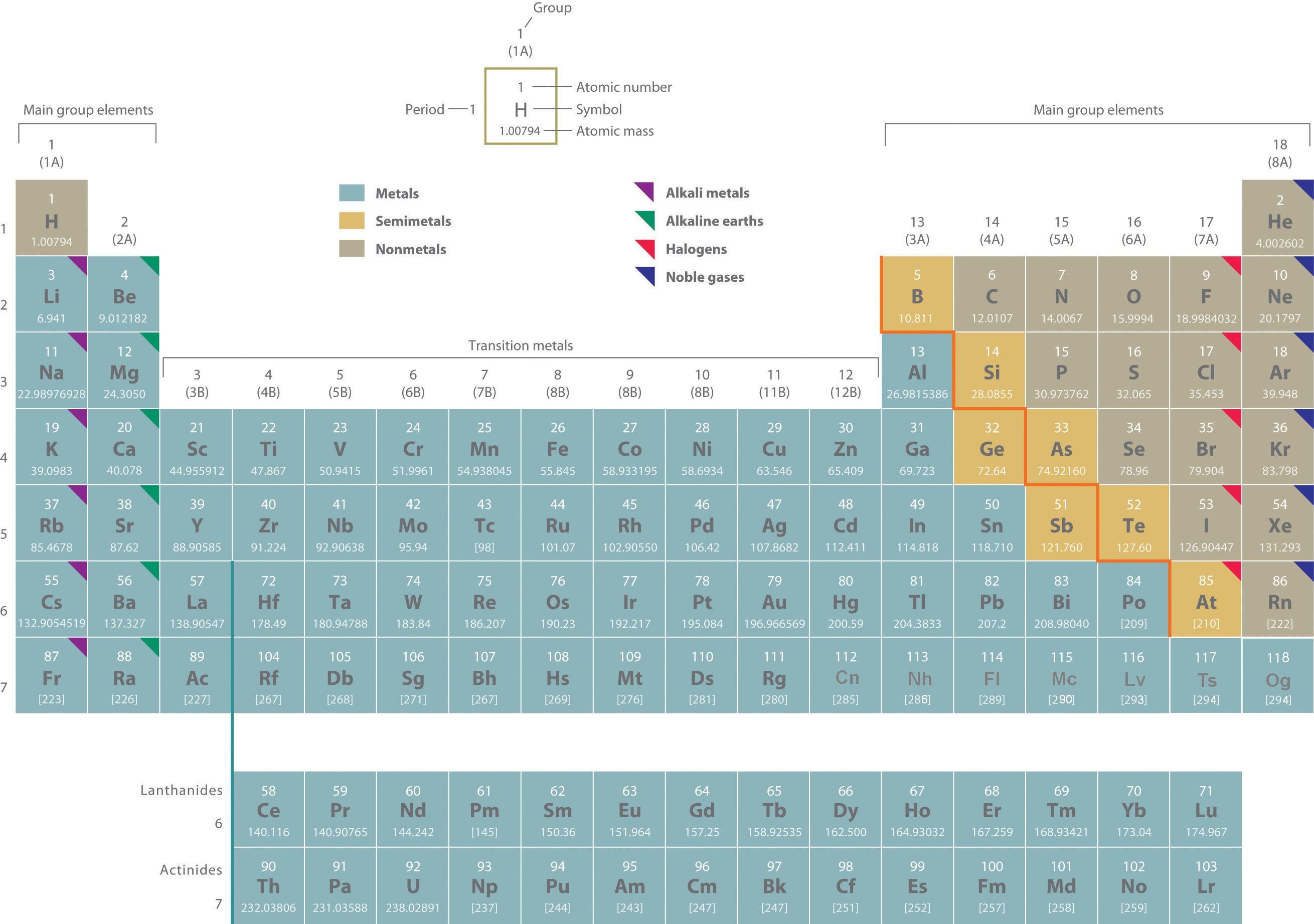
Two systems for numbering periodic groups are shown: 1–18 is the system currently recommended by the Inernational Union of Pure and Applied Chemistry (IUPAC); an older U.S. system, in which letters designate main group elements (A) and transition elements (B), is given parentheses.
An atomic mass in brackets indicates the mass of the longest-lived isotope of an element having no stable isotopes.
| List of Elements | |||
|---|---|---|---|
| Name | Symbol | Atomic Number | Atomic Mass |
| Actinium | Ac | 89 | [227]* |
| Aluminum | Al | 13 | 26.9815386(8) |
| Americium | Am | 95 | [243]* |
| Antimony | Sb | 51 | 121.760(1) |
| Argon | Ar | 18 | 39.948(1) |
| Arsenic | As | 33 | 74.92160(2) |
| Astatine | At | 85 | [210]* |
| Barium | Ba | 56 | 137.327(7) |
| Berkelium | Bk | 97 | [247]* |
| Beryllium | Be | 4 | 9.012182(3) |
| Bismuth | Bi | 83 | 208.98040(1) |
| Bohrium | Bh | 107 | [267]* |
| Boron | B | 5 | 10.811(7) |
| Bromine | Br | 35 | 79.904(1) |
| Cadmium | Cd | 48 | 112.411(8) |
| Calcium | Ca | 20 | 40.078(4) |
| Californium | Cf | 98 | [251]* |
| Carbon | C | 6 | 12.0107(8) |
| Cerium | Ce | 58 | 140.116(1) |
| Cesium | Cs | 55 | 132.9054519(2) |
| Chlorine | Cl | 17 | 35.453(2) |
| Chromium | Cr | 24 | 51.9961(6) |
| Cobalt | Co | 27 | 58.933195(5) |
| Copernicium† | Cn | 112 | [285]* |
| Copper | Cu | 29 | 63.546(3) |
| Curium | Cm | 96 | [247]* |
| Darmstadtium | Ds | 110 | [281]* |
| Dubnium | Db | 105 | [268]* |
| Dysprosium | Dy | 66 | 162.500(1) |
| Einsteinium | Es | 99 | [252]* |
| Erbium | Er | 68 | 167.259(3) |
| Europium | Eu | 63 | 151.964(1) |
| Fermium | Fm | 100 | [257]* |
| Flerovium | Fl | 114 | 289 |
| Fluorine | F | 9 | 18.9984032(5) |
| Francium | Fr | 87 | [223]* |
| Gadolinium | Gd | 64 | 157.25(3) |
| Gallium | Ga | 31 | 69.723(1) |
| Germanium | Ge | 32 | 72.64(1) |
| Gold | Au | 79 | 196.966569(4) |
| Hafnium | Hf | 72 | 178.49(2) |
| Hassium | Hs | 108 | [269]* |
| Helium | He | 2 | 4.002602(2) |
| Holmium | Ho | 67 | 164.93032(2) |
| Hydrogen | H | 1 | 1.00794(7) |
| Indium | In | 49 | 114.818(3) |
| Iodine | I | 53 | 126.90447(3) |
| Iridium | Ir | 77 | 192.217(3) |
| Iron | Fe | 26 | 55.845(2) |
| Krypton | Kr | 36 | 83.798(2) |
| Lanthanum | La | 57 | 138.90547(7) |
| Lawrencium | Lr | 103 | [262]* |
| Lead | Pb | 82 | 207.2(1) |
| Lithium | Li | 3 | 6.941(2) |
| Livermorium | Lv | 116 | 293 |
| Lutetium | Lu | 71 | 174.967(1) |
| Magnesium | Mg | 12 | 24.3050(6) |
| Manganese | Mn | 25 | 54.938045(5) |
| Moscovium | Mc | 115 | 290 |
| Meitnerium | Mt | 109 | [276]* |
| Mendelevium | Md | 101 | [258]* |
| Mercury | Hg | 80 | 200.59(2) |
| Molybdenum | Mo | 42 | 95.94(2) |
| Neodymium | Nd | 60 | 144.242(3) |
| Neon | Ne | 10 | 20.1797(6) |
| Neptunium | Np | 93 | [237]* |
| Nickel | Ni | 28 | 58.6934(2) |
| Nihonium | Nh | 113 | 286 |
| Niobium | Nb | 41 | 92.90638(2) |
| Nitrogen | N | 7 | 14.0067(2) |
| Nobelium | No | 102 | [259]* |
| Oganesson | Og | 118 | 294 |
| Osmium | Os | 76 | 190.23(3) |
| Oxygen | O | 8 | 15.9994(3) |
| Palladium | Pd | 46 | 106.42(1) |
| Phosphorus | P | 15 | 30.973762(2) |
| Platinum | Pt | 78 | 195.084(9) |
| Plutonium | Pu | 94 | [244]* |
| Polonium | Po | 84 | [209]* |
| Potassium | K | 19 | 39.0983(1) |
| Praseodymium | Pr | 59 | 140.90765(2) |
| Promethium | Pm | 61 | [145]* |
| Protactinium | Pa | 91 | 231.03588(2)* |
| Radium | Ra | 88 | [226]* |
| Radon | Rn | 86 | [222]* |
| Rhenium | Re | 75 | 186.207(1) |
| Rhodium | Rh | 45 | 102.90550(2) |
| Roentgenium | Rg | 111 | [280]* |
| Rubidium | Rb | 37 | 85.4678(3) |
| Ruthenium | Ru | 44 | 101.07(2) |
| Rutherfordium | Rf | 104 | [267]* |
| Samarium | Sm | 62 | 150.36(2) |
| Scandium | Sc | 21 | 44.955912(6) |
| Seaborgium | Sg | 106 | [271]* |
| Selenium | Se | 34 | 78.96(3) |
| Silicon | Si | 14 | 28.0855(3) |
| Silver | Ag | 47 | 107.8682(2) |
| Sodium | Na | 11 | 22.98976928(2) |
| Strontium | Sr | 38 | 87.62(1) |
| Sulfur | S | 16 | 32.065(5) |
| Tantalum | Ta | 73 | 180.94788(2) |
| Technetium | Tc | 43 | [98]* |
| Tellurium | Te | 52 | 127.60(3) |
| Tennessine | Ts | 117 | 294 |
| Terbium | Tb | 65 | 158.92535(2) |
| Thallium | Tl | 81 | 204.3833(2) |
| Thorium | Th | 90 | 232.03806(2)* |
| Thulium | Tm | 69 | 168.93421(2) |
| Tin | Sn | 50 | 118.710(7) |
| Titanium | Ti | 22 | 47.867(1) |
| Tungsten | W | 74 | 183.84(1) |
| Uranium | U | 92 | 238.02891(3)* |
| Vanadium | V | 23 | 50.9415(1) |
| Xenon | Xe | 54 | 131.293(6) |
| Ytterbium | Yb | 70 | 173.04(3) |
| Yttrium | Y | 39 | 88.90585(2) |
| Zinc | Zn | 30 | 65.409(4) |
| Zirconium | Zr | 40 | 91.224(2) |
| *Element has no stable isotope. A value enclosed in brackets, e.g. [209], indicates the mass number of the longest-lived isotope of the element. Three such elements (Th, Pa, and U), however, do have a characteristic terrestrial isotopic composition, and an atomic mass is given for them. An uncertainty in the last digit in the Atomic Mass column is shown by the number in parentheses; e.g., 1.00794(7) indicates ±0.00007. | |||
Source of data: Atomic weights of the elements 2001 (IUPAC Technical Report) as supplemented by the Table of Standard Atomic Weights 2005 (to be published in Pure and Applied Chemistry) on the IUPAC web site, and “Chart of the Nuclides” at https://www.nndc.bnl.gov/nudat2/.




