This is “Organic Nomenclature I: Alkanes, Cycloalkanes and Substituents”, section 4.12 from the book Principles of General Chemistry (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there.
4.12 Organic Nomenclature I: Alkanes, Cycloalkanes and Substituents
Learning Objectives
- To write IUPAC names for alkanes, cycloalkanes and alkyl halides.
- Given IUPAC systematic names of alkanes, cycloalkanes and alkyl halides write their structural formulas.
- To recongize structural isomers of organic compounds.
Organic chemists use a systematic nomenclature to indicate what atoms are present in a compound and how those atoms are connected in the molecule. Organic chemists can also indicate the atoms and how those atoms are connected with structural formulas. So, yes, there is redundancy in having both names and structures; however, imagine trying to describe a structure to another person over the phone, or while passing one another in the hallway, or in a text message. Also, if we only had structures, how would we search databases or order reagents? We can't always draw structures, sometimes we also need names.
The Parts
IUPAC names have different parts categorized as ending or prefix..
Ending: The ending of the name indicates the functional group present in the molecule. Since alkanes have only carbons and hydrogens connected by single bonds, there is no functional group to indicate and we end the name with -ane.
Prefix(es): Any number of prefixes can be applied to the ending of an alkane.
- The prefix immediately preceding the ending indicates the number of carbons in the parent chain.
- The prefix "cyclo" indicates a ring structure.
- Prefixes, usually accompanied by numbers, are used to indicate branches in the carbon chain.
- Prefixes, again usually accompanied by numbers, are used to indicate halogen atoms that have been substituted for hydrogens.
Note the Pattern
Every organic compound has a unique systematic name and a structure.
Prefix for the Number of Carbons in the Parent Chain
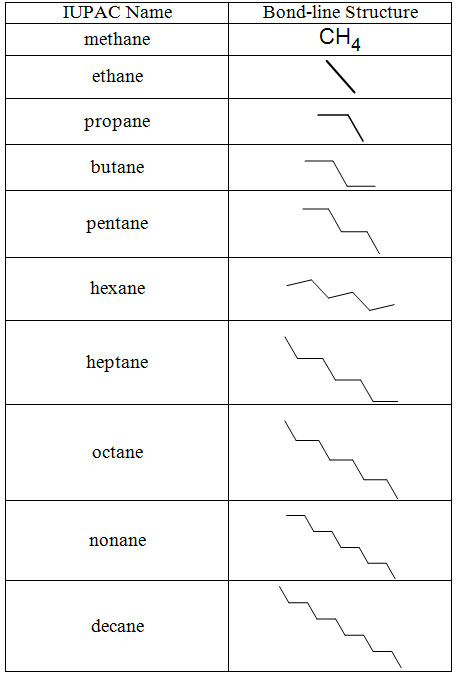
The parent chain is the longest continuous chain of carbon atoms. Below are the names of the first 10 alkanes with their bond-line structures. Note the suffix is -ane in all cases. The prefix indicates the number of carbons.
Table 4.7 The First Ten Alkanes
The Prefix "Cyclo"
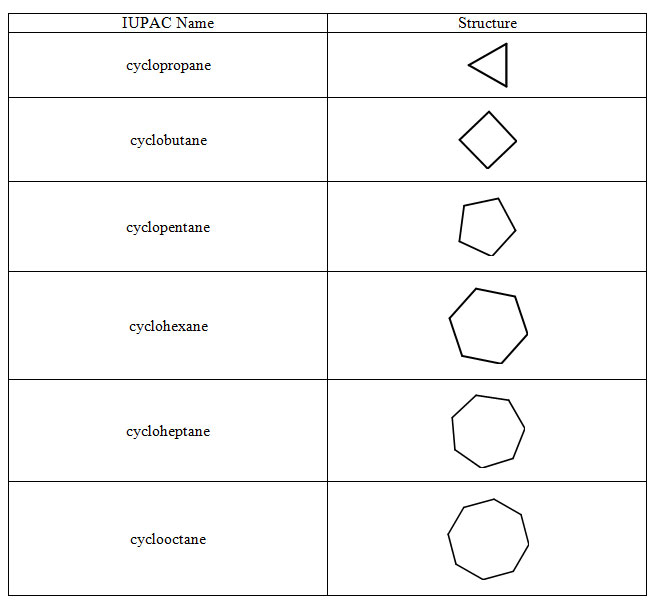
Rather than being a chain with two ends, alkanes can be ring structures. We use the same scheme as in table 4.7 for indicating the number of carbons in the ring. We include "cyclo" as a prefix to indicate the number of carbons in the ring.
Table 4.8 Cycloalkanes
Prefixes for Branches in the Parent Chain
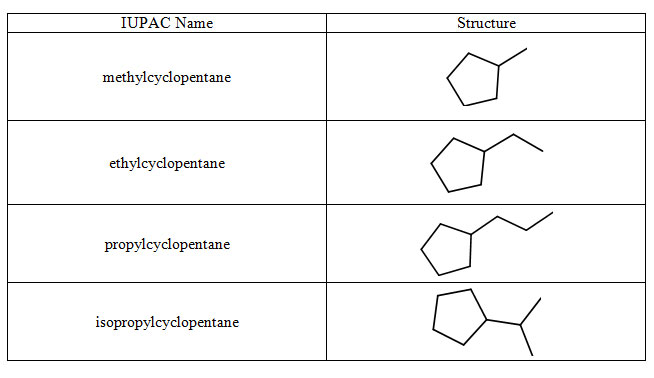
When a carbon is attached to more than two other carbons, we call this a branch point and this compound is a branched alkane. This means there are more carbons than just those in the parent chain. Below is a table of some alkyl groups which can be used as branches on parent chains. For the purpose of the example, the groups are attached to a cyclpentane ring. In fact, these groups can be attached to any carbon of any alkane.
Table 4.9 Alkyl Groups on a Cyclopentane Parent Chain
Branches are not limited to cyclic structures. Branches are also found on acyclic (not ring) structures. When the branches are on acyclic structures, one needs to take care when identifying the parent chain. Also, a number is required to indicate where the branch is in the structure. Consider the following examples.
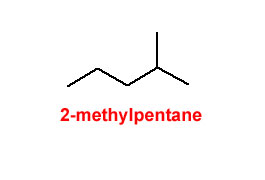
Figure 4.25 Methyl Groups on a 5-Carbon Chain

Beware of the rookie mistake often made when naming the rightmost structure. There is a tendency to try and name that rightmost structure as 1-methylpentane; however, the position of the methyl group at the end of the 5-carbon chain makes the parent chain (the longest continuous chain of carbon atoms) 6 carbons long.
While on the topic of numbering and rookie mistakes, another popular mistake when applying the numbers to the names of compounds is to forget the rule that the LOWEST number possible for designating a group on an alkane must always be used in the name. For example, consider the example in figure 4.26 (below).
Figure 4.26 The Lowest Number Possible is Always Used for Alkanes
The rookie mistake would be to name the compound 4-methylpentane.
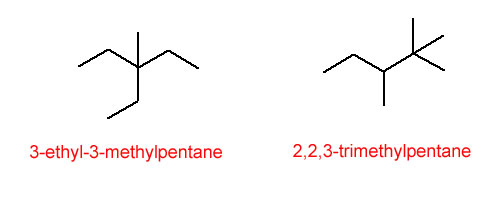
When more than one alkyl group is attached to the parent chain, the groups are listed alphabetically. Also, if more than one of the same branch are present, the greek prefixes from section 2.4 are applied. See the examples below:
Figure 4.27 The Lowest Number Possible is Always Used for Alkanes
Structural Isomers
As you dive deeper into chemistry, you'll find several different types of isomers. Structural isomers (sometimes called constitutional isomers) are different compounds that have the same molecular formula but a different connectivity of the atoms. The compounds in figure 4.25 represent some structural isomers with the molecular formula C6H14. The compounds in figure 4.27 represent a pair of structural isomers with the molecular formula C8H18.
Prefixes Indicating the Presence of a Halogen
The prefixes fluoro-, chloro-, bromo- and iodo- are used to indicate the substitution of a halogen atom for one of the hydrogens on the carbon chain. These compounds are sometimes called alkyl halides or haloalkanes.
Figure 4.28 Some Alkyl Halides
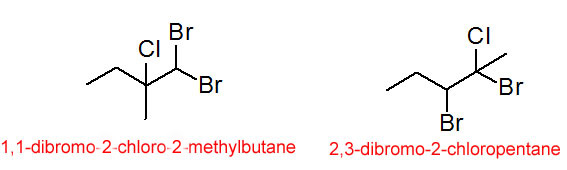
(Note that the compounds in figure 4.28 represent a pair of structural isomers.)
This section is taking you one giant step forward in the nomenclature associated with organic compounds. If you want to dive deeper, the International Union of Pure and Applied Chemists has given permission to publish their 1993 Nomenclature of Organic Compounds to ACDlabs. You can access the html version at the address:
http://www.acdlabs.com/iupac/nomenclature/
The IUPAC recommendations can be a little onerous for beginners and practiced users alike. When structures or names get very complicated, many chemists employ a program like MarvinSketch to assign a name to the structure. Likewise MarvinSketch can draw the structure by simply pasting the text name into the workspace.
Numerical Problems
-
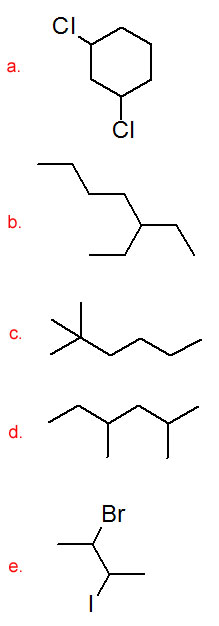
Draw a structural formula for each compound.
- 1,3-dichlorocyclohexane
- 3-ethylheptane
- 2,2-dimethylhexane
- 2,4-dimethylhexane
- 2-bromo-3-iodobutane
-
Draw a structural formula for each compound.
- 2,2-4-trimethylpentane
- 2,5-dimethylhexane
- 3-ethyl-2-methylpentane
- 2,2,3-trimethylpentane
- 2,2,3,3-tetramethylbutane
-
Below is a list of incorrect names. Write the correct name for each compound.
- 2-ethylbutane
- 3-propylpentane
- 4-bromopentane
- 2-chloro-3-bromohexane
- 2,3-dimethylcyclohexane
-
Name each compound.
- CH3CH2CH(CH3)CH2CH2CH3
- CH3CH2CH(CH3)2
- CH3CH2CHBrCH2CH3
- CH3CH2CBr3
- CH3CCl2CH2CH3
-
Name each compound.
-
-
Draw the structure of each compound.
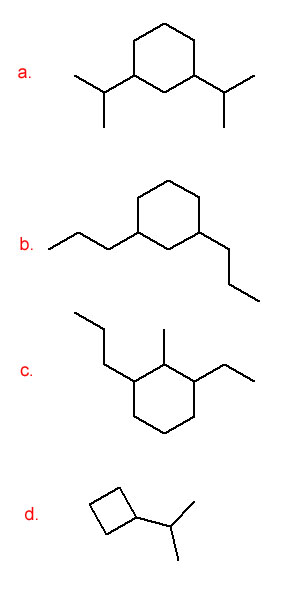
- 1,3-diisopropylcyclohexane
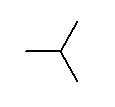
- 1,3-dipropylcyclohexane
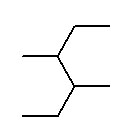
- 1-ethyl-2-methyl-3-propylcyclohexane
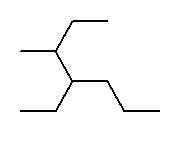
- isopropylcyclobutane
Answers
-
-
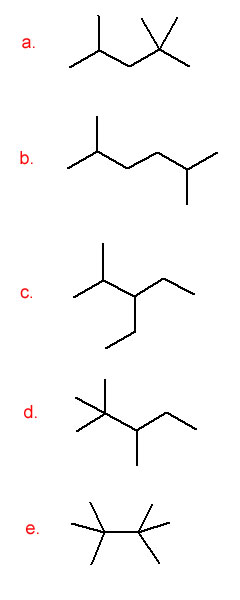
-
- 3-methylpentane
- 3-ethylhexane
- 2-bromopentane
- 3-bromo-2-chlorohexane
- 1,2-dimethylcyclohexane
-
- 3-methylhexane
- 2-methylbutane
- 3-bromopentane
- 1,1,1-tribromopropane
- 2,2-dichlorobutane
-
- 2-methylpropane
- 3,4-dimethylhexane
- 4-ethyl-3-methylheptane
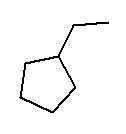
- ethylcyclopentane
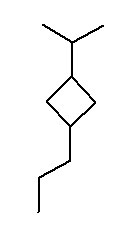
- 1-isopropyl-3-propylcyclopropane
-