This is "Conversion Factors", section 1.11 added to the book Principles of General Chemistry (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there.
1.11 Conversion Factors
Learning Objective
- To learn how to solve problems using dimensional analysis.
A powerful approach to solving problems in chemistry goes by a variety of names. The conversion factor approach is also called dimensional analysis, or factor-label problem solving. In this section we will use conversion factors to solve metric-metric, metric-english and density problems. As we move through the material we will see more and more applications for conversion factors with increasing level of complexity. It's best to start using the conversion factor approach now while the material is simple. If you master the conversion factor approach now, while the problems are easy, when the complexity increases, it's just a matter of plugging in new conversion factors. At the conclusion of every chapter, you should ask yourself if you learned any new conversion factors for this section. You should be able to answer "yes" for nearly every chapter.
Metric-Metric Conversions
In Essential Skills 1 , the metric system base units and prefixes were detailed. We can use the information in Table 1.8 as conversion factors to change units within the metric system. Mathematically speaking we are simply multiplying a quantity by the number 1. But we are writing the number 1 as a factor such that the undesired units cancel leaving the desired unit. For example, if we were wanted to cook 10 quarter pound hamburgers and we wanted to know how many pounds of ground beef required, we would set up the following-
Since 1 pound is equal to 4 burgers, the quantity "1 pound / 4 burgers" is the number 1. Likewise the quantity "4 burgers / 1 pound" is the number 1; however, had we used "4 burgers / 1 pound" as the factor, the units would not have canceled. This problem illustrates the importance of including units in our conversion factors. Had we just written "1 / 4" or "4 / 1," we would not be multiplying by one. In the fomer case we would have got the correct number, but the units would have been incorrect. In the latter case we would have calculated both the wrong value AND the wrong units. To use the conversion factor method of problem solving you must always include the units in your calculations.
For doing the calculations within the metric system we often face several possibilities for conversion factors. Table 1.8 says 1 μm = 1 × 10-6 m. It is often more convenient however to follow the general rule to avoid (-) powers in conversion factors. Mathematically, it doesn't matter but in terms of the workings of the human mind, rather than saying 1 μm = 1 × 10-6 m, instead say 1 m = 1 × 106 μm. These are equivalent statements. Mentally, it makes the calculation simpler if you avoid the negative exponents.
Example 7
Convert 75.2 nm to μm
Given: a metric measurement of length
Asked for: different metric unit
Strategy:
Apply the prefixes from Table 1.8 and and arrange arrange units such that the given unit cancels leaving the desired unit.
Solution:
Exercise
Convert 0.00572 km to cm.
Answer: 572 cm
English-Metric Conversions
Through the centuries Americans have become familiar with an English system of units. So, as American chemists, we need to be able to convert between English and metric units.
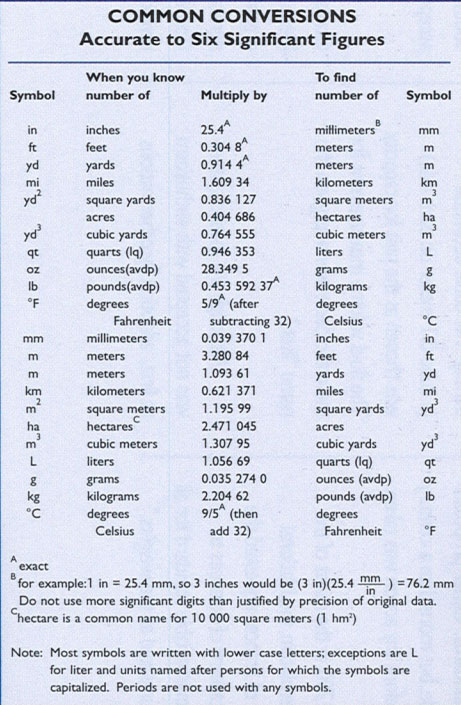
Table of Conversion Factors Courtesy of the National Institute of Standards and Technology (NIST).
It is not necessary or even desirable to memorize all of the factors in the above table. You will however need to know at least three factors from memory: one factor for mass, one factor for volume and one factor for length. One of each is all that is required assuming you know your metric prefixes from Table 1.8 in Essential Skills 1.
454 g = 1 lb
1.06 qts = 1 L
2.54 cm = 1 inch
The numerical exercises below demonstrate lots of examples of how to use these factors. Hopefully you see the problem-solving process for the English-metric conversions iis exactly the same as for the metric-metric conversions with the added spice of a few non-decimal factors.
One of the big advantages of using this conversion factor approach to problem solving is that you can easily look back at your work and see if you have made any mistakes. Just cross off your units and ask yourself if each factor is equal to the number 1. If the units and factors check out, your answer is correct, assuming your arithmetic is correct.
Note the Pattern
All conversion factors are equalities arranged in form such that the original units cancel.
Summary
Conversion factors are applied to change units.
Key Takeaway
- Of course we know inches and cm are related. Of course we know pounds and grams are related, etc. As we move through the course, we will see more and more of nature's behavior can be related through conversion factors.
Numerical Problems
Courtesy of Fred Redmore, Highland Community College
-
Carry out the following conversions.
- 42 cm to m
- 250 mL to L
- 45 μg to g
- 0.12 m to km
- 0.015 cm3 to L
- 1.2 m to cm
- 0.15 L to mL
- 6.2 kg to g
- 1.2 m to μm
- 4.5 L to cm3
-
Carry out the following conversions.
- 25.2 cm to in.
- 42.5 g to lb
- 2.4 in. to cm
- 82.5 qt to L
- 0.15 lb to g
- 6.50 in. to cm
- 8.50 lb to g
- 10.5 cm to in.
- 7.2 L to qt
- 67 g to lb
-
Carry out the following conversions.
- 1.5 cm to mm
- 12.8 mg to kg
- 95 mL to μL
- 0.23 km to cm
- 86 mm to km
- 47.2 μL to mL
- 6.3 kg to mg
- 0.825 cm to mm
-
Carry out the following conversions.
- 85 cm to ft
- 0.245 qt to mL
- 3.2 kg to lb
- 67.5 in. to m
- 0.12 lb to μg
- 845 ft to km
- 6.2 lb to kg
- 0.452 m to in.
- 863 mg to lb
- 1.30 ft to cm
- 7.50 μL to qt
- 85.2 km to ft
-
Perform the following conversions:
- If a car is traveling at a rate of 100 km/hr, how many meters will it travel in 1.0 minute?
- If a person walks at a rate of 3.5 mi/hr, how many minutes will it take to walk 5.8 km? (1 km = 0.62 mi)
- If a car is traveling at a rate of 55 mi/hr, how many cm will it travel in 1.0 minute? (1 km = 0.62 mi)
- If you are riding a bicycle at a rate of 15 km/hr, how many minutes will it take to travel 6.25 m?
Answers
-
- (42 cm)= 0.42 m
- (250 mL)= 0.25 L
- (45 μg)= 45 × 10-6 g = 4.5 × 10-5 g
- (0.12 m)= 0.12 × 10-3 km = 1.2 × 10-4 km
- (0.015 mL)= 0.015 × 10-3 L = 1.5 × 10-5 L
- (1.20 m)= 120 cm
- (0.15 L)= 150 mL
- (6.2 kg)= 6200 g
- (1.2 m)= 1.2 × 106 μm
- (4.5 L)= 4.5 × 103 cm3
-
(25.2 cm)= 9.92 in.
- (42.5 g)= 0.0936 lb
- (2.4 in)= 6.1 cm
- (82.5 qt)= 77.8 L
- (0.15 lb)= 68.1 g
- (6.50 inches)= 16.5 cm
- (8.50 lb)= 3.86 × 103 g
- (10.5 cm)= 4.13 in.
- (7.2 L)= 7.6 qt
- (67 g)= 0.15 lb
-
(1.5 cm)= 15 mm
- (12. mg)= 12.8 × 10-6 kg = 1.28 × 10-5 kg
- (95 mL)= 95 × 103 μL = 9.5 × 104 μL
- (0.23 km)= 0.23 × 105 cm = 2.3 × 104 cm
- (86 mm)= 86 × 10-6 km = 8.6 × 10-5 km
- (47.2 μL)= 47.2 × 10-3 mL = 0.0472 mL
- (6.3 kg)= 6.3 × 106 mg
- (0.825 cm)= 8.25 mm
-
(85 cm)= 2.8 ft
- (0.245 qt)= 231 mL
- (3.2 kg)= 7.0 lb
- (67.5 in)= 1.71 m
- (0.12 lb)= 5.5 × 107 μg
- (845 ft)= 0.258 km
- (6.2 lb)= 2.8 kg
- (0.452 m)= 17.8 in.
- (863 mg)= 1.90 × 10-3 lb
- (1.3 ft)= 4.0 × 101 cm
- (7.5 μL)= 8.0 × 10-6 qt
- (85.2 km)= 2.80 × 105 ft
-
(1 min.)= 1.7 × 103 m
- (5.8 km)= 62 minutes
- (1 min)= 1.5 × 105 cm
- (6.25 m)= 0.025 min




